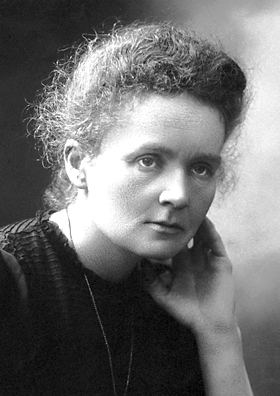 Marie Curie, the woman who discovered radioactivity, found and named two elements and became the only scientist in history to win Nobel Prizes in two different sciences recently came in at the top of a BBC poll on the 100 most influential women in history. In her honor, here is a brief excerpt about her and her groundbreaking work from my book They Called Me Mad: Genius, Madness and the Scientists Who Pushed the Outer Limits of Knowledge.
Marie Curie, the woman who discovered radioactivity, found and named two elements and became the only scientist in history to win Nobel Prizes in two different sciences recently came in at the top of a BBC poll on the 100 most influential women in history. In her honor, here is a brief excerpt about her and her groundbreaking work from my book They Called Me Mad: Genius, Madness and the Scientists Who Pushed the Outer Limits of Knowledge.
“When she recovered, Marie began working on her doctoral thesis. Wilhelm Conrad Röntgen had recently discovered X-rays. A short time later, Antoine-Henri Becquerel accidentally discovered something similar being given off by uranium salts. He shamelessly named them Becquerel rays, but then abandoned research when he found them too difficult to measure.
Marie decided to take up where Becquerel had left off. Initially she didn’t have much success measuring the strength of the rays until Pierre modified and improved his electroscope. Such devices were so notoriously difficult to work with that the scientist Lord Rayleigh once wrote all electrometers were “designed by the devil.” Undeterred, Marie spent day after day laboriously measuring samples of pulverized uranium.
 Once Marie had established the strength of the rays given off by pure uranium, she used that as a standard against which to measure other substances. She measured samples of the element thorium and found that they gave off rays too, although not as strongly as the uranium. She then measured a type of ore known as pitchblende. It was a heavy, black ore from which uranium was extracted. Surprisingly, Marie found that even after the extraction, the pitchblende continued to emit rays. In fact the rays she detected coming from the pitchblende were stronger than the rays from pure uranium.
Once Marie had established the strength of the rays given off by pure uranium, she used that as a standard against which to measure other substances. She measured samples of the element thorium and found that they gave off rays too, although not as strongly as the uranium. She then measured a type of ore known as pitchblende. It was a heavy, black ore from which uranium was extracted. Surprisingly, Marie found that even after the extraction, the pitchblende continued to emit rays. In fact the rays she detected coming from the pitchblende were stronger than the rays from pure uranium.
Intrigued, she began exhaustively looking at other elements and compounds to determine if they gave off similar rays and if so at what strength. By April of 1898 she had reached a startling conclusion. She wrote up her findings in a formal paper, but since neither she nor Pierre were members of the Academy of Sciences, her professor, Gabriel Lippmann presented the paper in her stead:
It was necessary at this point to find a new term to define this new property of matter manifested by the elements of uranium and thorium. I proposed the word radioactivity.
During the course of my research, I had occasion to examine not only simple compounds, salts and oxides, but also a great number of minerals. Certain ores containing uranium and thorium proved radioactive, but their radioactivity seemed abnormal, for it was much greater than…I had been led to expect. This abnormality greatly surprised us. When I had assured myself that it was not due to an error in the experiment, it became necessary to find an explanation. I then made the hypothesis that the ores of uranium and thorium contain in small quantity a substance much more strongly radioactive than either uranium or thorium itself. This substance could not be one of the known elements, because these had already been examined; it must therefore, be a new chemical element. (Goldsmith)
As the scientist Frederick Soddy later put it, “Pierre Curie’s greatest discovery was Marie Sklodowska. Her greatest discovery was…radioactivity.” She had not only discovered and named an entirely new property of atoms, but had postulated that it could be used to find new elements. Shortly after Marie’s paper was presented, Pierre quit his work with crystals to assist her full-time. Other scientists became interested, including Becquerel. The race to discover these new elements was on.
Soon Marie was competing against scientists in Germany, Italy and England. She managed to isolate a substance from the pitchblende that behaved like the element bismuth, but was 17 times more radioactive than pure uranium. As she continued to purify her samples, the radioactivity increased. Within two weeks she had produced samples 150 times as radioactive, then 300 times as radioactive, and finally 400 times as radioactive as pure uranium. Marie was confident that she had discovered an entirely new element, and she named it in honor of her beloved Polish homeland polonium.
 She didn’t stop there. By December, she found a second substance in the pitchblende that behaved like barium. This one was 900 times as radioactive as pure uranium. Believing she had discovered yet another new element, she wanted to confirm her results. One of her colleagues at EPCI, Eugene Demarcay was using a method called spectroscopy. It consisted of heating an element until it became a glowing gas and then using a prism to refract the light given off. The light would form a rainbow pattern or spectra, and each element gave off a unique pattern. On December 19, 1898, Demarcay tested Marie’s new sample and confirmed she had discovered a new element. She named it using the Latin word for ray, radius, and christened the new element radium.
She didn’t stop there. By December, she found a second substance in the pitchblende that behaved like barium. This one was 900 times as radioactive as pure uranium. Believing she had discovered yet another new element, she wanted to confirm her results. One of her colleagues at EPCI, Eugene Demarcay was using a method called spectroscopy. It consisted of heating an element until it became a glowing gas and then using a prism to refract the light given off. The light would form a rainbow pattern or spectra, and each element gave off a unique pattern. On December 19, 1898, Demarcay tested Marie’s new sample and confirmed she had discovered a new element. She named it using the Latin word for ray, radius, and christened the new element radium.
Although Marie and Pierre were certain of her results, others were unsure. The physics community was relatively comfortable with theoretical discoveries, but the chemistry community demanded something more tangible. To convince them, the Curies would need to isolate enough of the new elements to actually be measured and tested. That would prove to be a monumental task.
Pierre applied for laboratory space at the Sorbonne. He was turned down. He then applied at the EPCI, but the best they could provide was an abandoned hangar that had formerly been used by students to perform human dissections in. It had an asphalt floor and a leaky roof, and was in such deplorable condition, that it was no longer suitable to house the cadavers. This was where Marie and Pierre carried out their great work.
The next hurtle was to acquire the vast amounts of pitchblende necessary to isolate a usable amount of the new elements. Fortunately, Pierre learned that there was a mountain of pitchblende sitting in a waste pile in the woods of St. Joachimsthal, then part of Austria. The uranium had already been extracted and the piles of pitchblende sludge were simply dumped there. The Austrians were only too happy to get rid of what they considered waste material, so the Curies only needed to come up with enough money to pay for its transport. A wealthy philanthropist, the Baron Edmond de Rothschild anonymously provided a modest donation for the purpose.
 Once the first few tons of pitchblende arrived in the courtyard outside the hangar, the real back-breaking work could begin. It quickly became apparent that radium would be the easier of the two elements to extract, so the Curies focused on that. They divided the work, Pierre concentrating on the physics and determining its physical properties, and Marie on the chemistry of the extraction process. She carried out an exhaustive series of successive cleanings and boilings of the pitchblende in great twenty kilogram [forty-four pound] batches, stirring the enormous pots with an iron rod almost as tall as she was. She then used chemical processes to purify it further, and crystallize the residues. Initially, she had thought that radium made up approximately one percent of the pitchblende. It turned out that the actual figure was closer to one millionth of one percent. It took her thousands of purifications and almost seven tons of pitchblende to produce a single gram of radium.
Once the first few tons of pitchblende arrived in the courtyard outside the hangar, the real back-breaking work could begin. It quickly became apparent that radium would be the easier of the two elements to extract, so the Curies focused on that. They divided the work, Pierre concentrating on the physics and determining its physical properties, and Marie on the chemistry of the extraction process. She carried out an exhaustive series of successive cleanings and boilings of the pitchblende in great twenty kilogram [forty-four pound] batches, stirring the enormous pots with an iron rod almost as tall as she was. She then used chemical processes to purify it further, and crystallize the residues. Initially, she had thought that radium made up approximately one percent of the pitchblende. It turned out that the actual figure was closer to one millionth of one percent. It took her thousands of purifications and almost seven tons of pitchblende to produce a single gram of radium.
Working tirelessly, day after day, under the most abominable of conditions, the Curies were at their best. As Marie describe the time:
We were very happy in spite of the difficult conditions under which we worked. We passed our days at the laboratory, often eating a simple student’s lunch there. A great tranquility reigned in our poor, shabby hangar; occasionally, while observing an operation, we would walk up and down talking of our work, present and future. When we were cold, a cup of hot tea, drunk beside the stove, cheered us. We lived in a preoccupation as complete as that of a dream.
One evening, after tucking Irene into bed, Marie and Pierre returned to the laboratory to continue their work. As they stepped into the darkened hangar, they saw a faint glow upon the shelves. It was the jars of radium salts that Marie had been purifying. Each contained only a few grains of the precious radium, but it was enough to make them glow in the dark. They reminded Marie of “faint, fairy lights,” and she described her and Pierre’s reaction, “From all sides we could see their slightly luminous silhouettes, and these gleaming, which seemed suspended in the darkness, stirred us with ever new emotion and enchantment.” (Curie, Pierre Curie)
They were blissfully unaware that the radiation responsible for those enchanting lights was also taking a heavy toll on their health. Pierre began suffering from excruciating pain in his legs. He thought it was rheumatism, and attributed it to the dampness in the hangar. His fingers became hard and cracked from handling the radium salts. Marie began to lose weight. Her doctors suspected that she had come down with tuberculosis, the disease which had killed her mother, and recommended rest and country air. She ignored them.
After four years, all of their suffering was rewarded. Marie was able to produce a tenth of a gram of radium. It was a minuscule amount, the equivalent of a few grains of sand, but it was enough for the Curies to determine its atomic weight and place it correctly as number 88 on Mendeleev’s periodic table. Like Priestley before them, they shared their knowledge freely and would soon send samples of radium even to competing scientists like Ernest Rutherford, Frederick Soddy and Great Brittan’s Lord Kelvin.”